Automated handling of critical data
Goodbye to scattered or incomplete GxP data.
With TRACKS, manufacturers within heavily regulated industries can stay on top of who does what on which machine or component - at all times.

All critical data in one place
Decentralized logging in each level 1 or 2 component and an abundance of paper forms cause many manufacturers to struggle with scattered or incomplete GxP data.
By collecting critical GxP data from components, machines, production lines or entire facilities and centralizing it in one system, TRACKS help manufacturers avoid this problem.
With TRACKS they can:
- Check if they are out of compliance because a critical parameter has been changed.
- Minimize time spent on manual document handling around FAT/SAT and batch changeovers.
- Speed up revalidation by being able to instantly identify which parameters have changed.
- Quickly return to machine baseline settings if performance drops due to parameter changes.
- Have documentation at hand for audits and proactive process analysis/improvement.
Explore features and functionality here
TRACKS integrates with your Active Directory to handle all users and access rights.
Access rights are role-based and support users having different roles on different machine groups.
Authentication and authorization is centralized in TRACKS.
Audit trails are generated immediately in TRACKS whenever any electronic records, such as parameters, are changed.
TRACKS also supports the storage of audits from other systems and offers custom importers and exporters, for seamless integration with external systems. Standard importers and exporters exist for XML, JSON, SQL servers, OPC UA, and CSV files.
Audits adhere to regulations and include at least the following:
- Timestamps with date, time, and time zone.
- Clear, legible, and indelible records.
- Information about who made the changes.
Events such as authentication and alarms are automatically saved in the audit trail.
All audit table columns can be easily sorted, filtered, grouped, and saved in physical formats such as PDF or CSV.
Parameters in TRACKS are data obtained from other systems.
TRACKS retains a full history of all parameters, ensuring a detailed record of changes over time.
Parameters offers the following features:
- Monitoring: Parameters can be marked as critical for easy monitoring.
- Automatic Audit Trails: All changes to parameters are automatically logged into the audit trail.
- Custom importers and exports: The system provides a standard interface for third-party systems to enable custom import and export of data.
TRACKS allows users to create baselines, which are snapshots capturing the state of all parameters at a specific moment. Users can easily view or compare these baselines within the application.
Parameters across the system can be compared at any two given points in time, facilitating easy comparison during Factory Acceptance Tests (FAT) or Site Acceptance Tests (SAT).
When comparing baselines, new parameters, changes, and deleted parameters are color-coded. Baselines can also be exported to physical formats such as PDF or CSV.
Audit data and parameters can be easily sorted, filtered, grouped, and saved in physical formats such as PDF or CSV.
The Equipment module in TRACKS serves as a platform for overview and maintenance of testing equipment, which includes devices monitored for service and calibration.
Additionally, the system provides functionality for creating equipment lists for tests and other related activities.
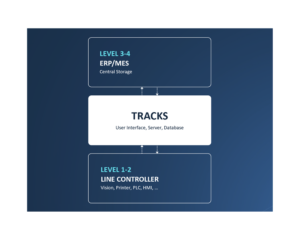
TRACKS is the backbone in any CIM solution.
All CIM solutions rely on TRACKS for user management, audit trails and more.
Acting as a smart bridge between machine components at level 1 and the Enterprise System at level 3, TRACKS allows for efficient, centralized user management and monitoring of critical parameters.
This makes our solutions ideal for pharma and other industries with high standards for electronic documentation and compliance.
Want to centralize tracking of GxP data?