Compliance with cim tracks
Keep Track and Stay Compliant
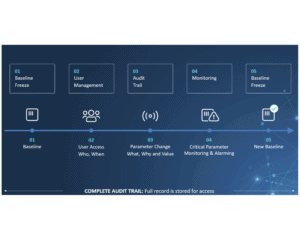
For production companies, keeping track of the activities on assembly lines and packaging lines is key to staying compliant with international standards and regulations. This is especially true for the pharma industry, where the CFR 21-part 11 regulation sets high standards for electronic documentation and signatures. Failure to comply means failure to operate, and that is devastating to business. So choosing the right audit trail system is more important than ever.
CIM TRACKS acts as a smart bridge between machine components at level 1 and the Enterprise System at level3 and allows you to monitor critical parameters and perform user management much more efficiently.